Water Vapor Pressure Chart
Water Vapor Pressure Chart. The vapour pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume.
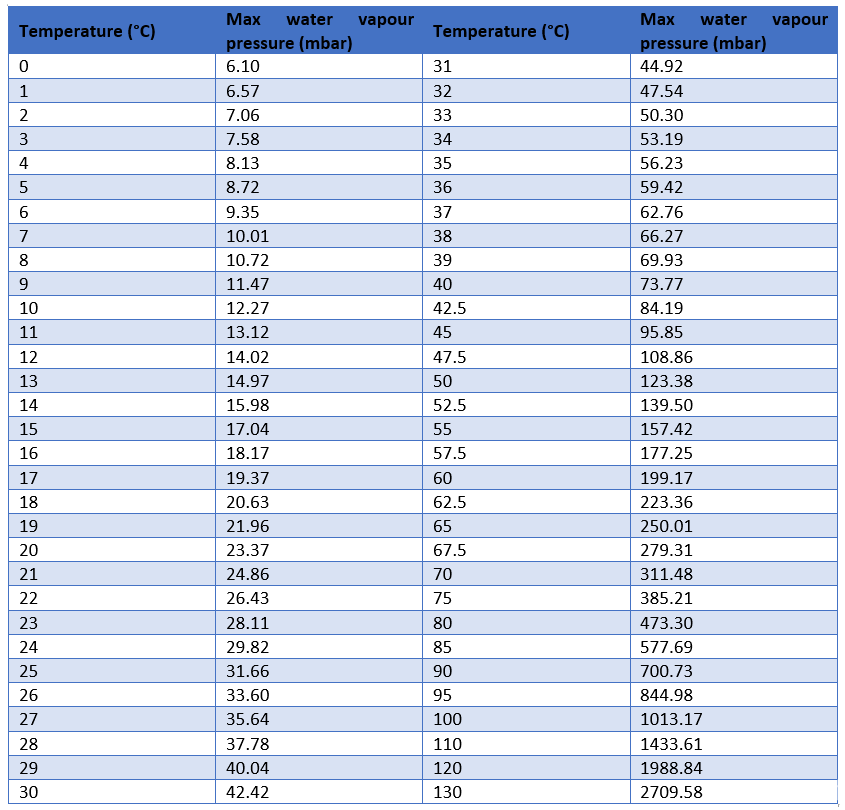
The pressures are stated in mega-Pascals, where a Pascal is a Newton per square meter, and as a multiple of standard atmospheric pressure.
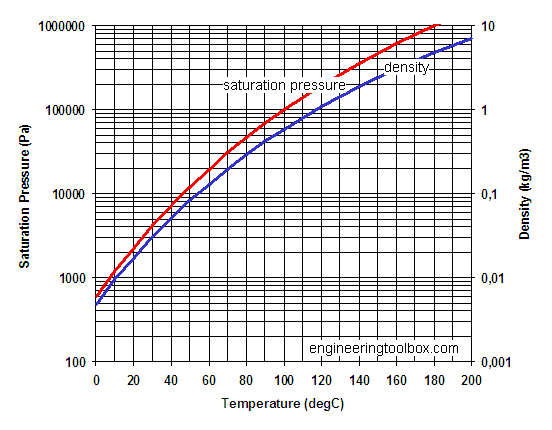
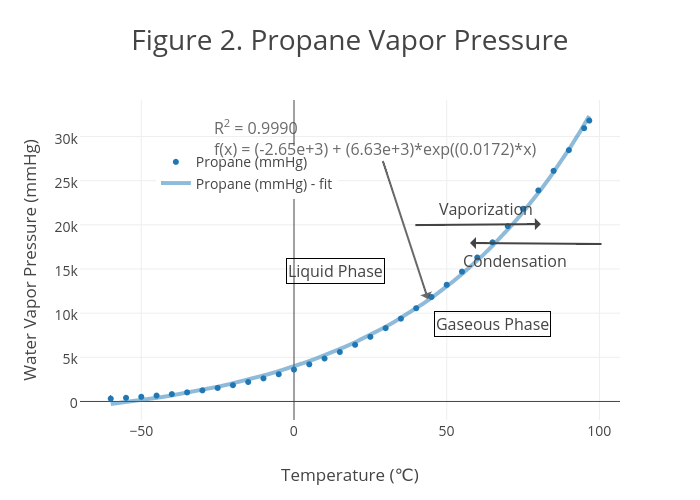
This is illustrated in the vapor pressure chart (see right) that shows graphs of the vapor pressures versus temperatures for a variety of liquids.
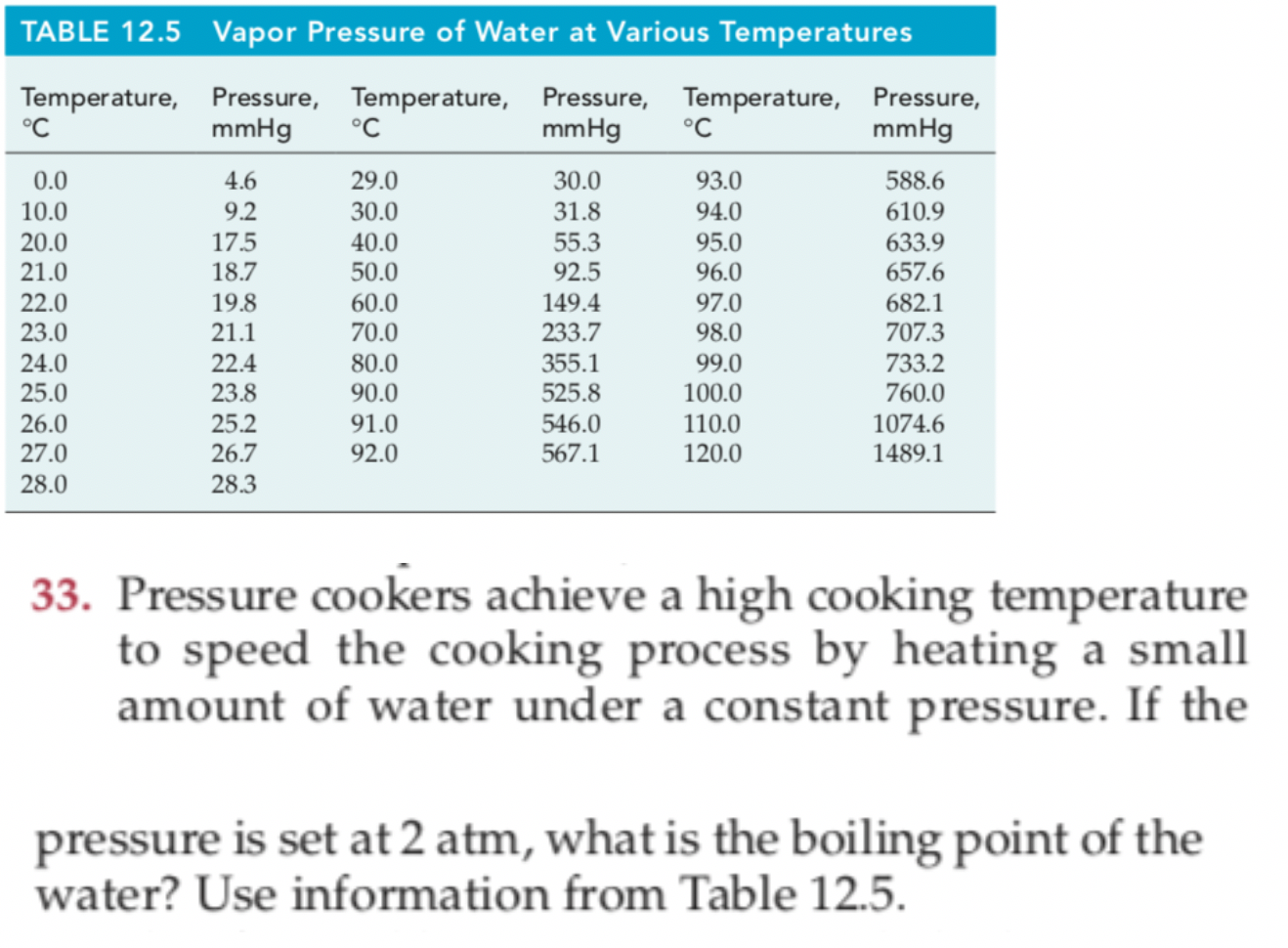
One of the most referenced set of data in lyophilization is the vapor pressure of ice chart. As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points. The pressure exerted by the vapor phase is called the. vapor or saturation pressure.
Rating: 100% based on 788 ratings. 5 user reviews.
Ronald Farrel
Thank you for reading this blog. If you have any query or suggestion please free leave a comment below.

0 Response to "Water Vapor Pressure Chart"
Post a Comment